The main component of Turmeric is CURCUMIN and its effectiveness has been demostrated in serveral clinical trials. Despite its effects, curcumin-based drugs -and diet supplements- are not readily available in the market because of their low bioavailability.
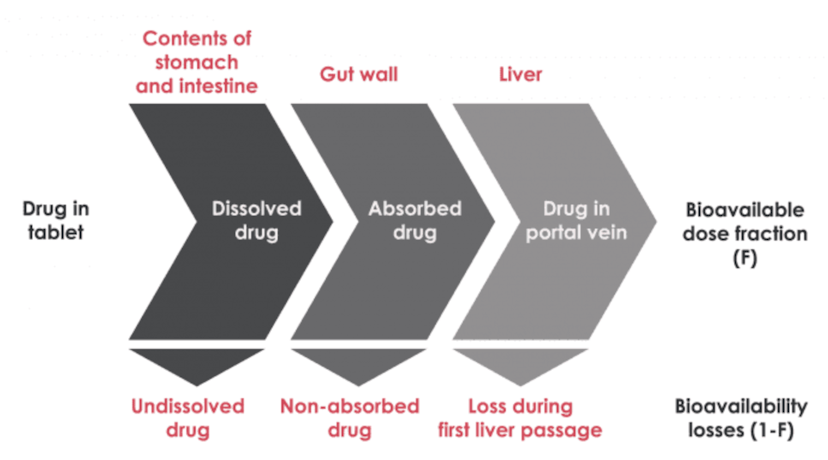
Bioavailability
Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. In simple words, bioavailability means how much our body is capable of absorbe and take advantage of the benefits of certain drugs or supplements.Causes of low bioavailability
- Size of the molecule - Orally administered drugs must pass through the intestinal wall and then the portal circulation to the liver; both are common sites of first-pass metabolism (metabolism that occurs before a drug reaches systemic circulation). Thus, many drugs may be metabolized before adequate plasma concentrations are reached. Low bioavailability is most common with oral dosage forms of poorly water-soluble, slowly absorbed drugs.
- Insufficient time of absroption - Insufficient time for absorption in the gastrointestinal (GI) tract is a common cause of low bioavailability. If the drug does not dissolve readily or cannot penetrate the epithelial membrane (eg, if it is highly ionized and polar), time at the absorption site may be insufficient. In such cases, bioavailability tends to be highly variable as well as low.
- Chemical reactions - They include formation of a complex (eg, between tetracycline and polyvalent metal ions), hydrolysis by gastric acid or digestive enzymes (eg, penicillin and chloramphenicol palmitate hydrolysis), conjugation in the intestinal wall (eg, sulfoconjugation of isoproterenol), adsorption to other drugs (eg, digoxin to cholestyramine), and metabolism by luminal microflora.
- Poor water solubility - Curcumin is an oil soluble pigment, practically insoluble in water at acidic and neutral pH, soluble in alkali. It is stable at high temperatures and in acids, but unstable in alkaline conditions and in the presence of light.
- Other factors - Age, sex, physical activity, genetic phenotype, stress, disorders (eg, achlorhydria, malabsorption syndromes), or previous GI surgery (eg, bariatric surgery) can also affect drug bioavailability.
- Conclusion - According to The Nutraceutical Bioavailability Classification Scheme (NuBACS), physicochemical/physiological factors contributing to the poor bioavailability (BA) of bioactive compounds -such as curcumin- can be categorized in five classes:
- Bioaccessibility (B*)
- Absorption (A*)
- Transformation (T*)
- Distribution (D*)
- Excretion (E*)

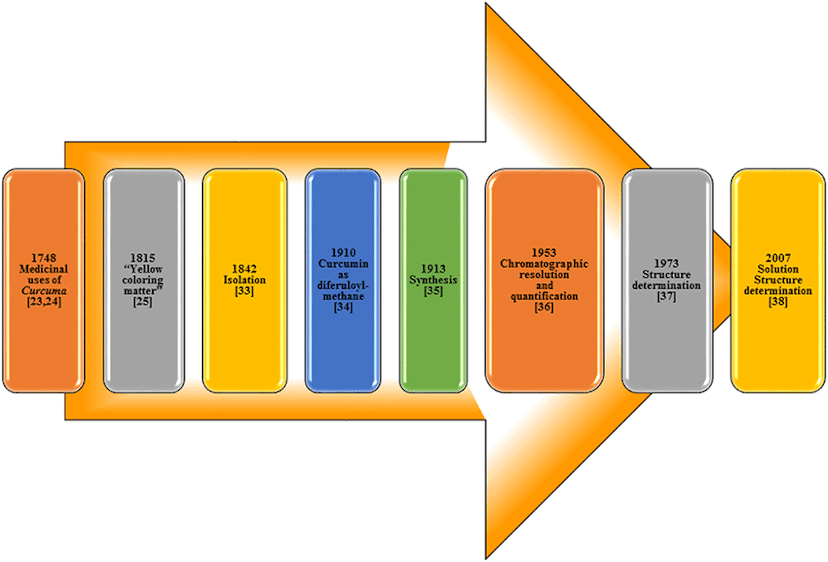
Milestones in curcumin bioavailability
Unformulated curcumin
In 1978 was reported for the first time poor amount of curcumin in blood plasma of rats after oral administration. Same type of experiments in humans revealed that curcumin was practically undetectable in blood stream. Intravenous administration was also tried showing better -but not significant- absorption rates.Nanocurcumin
Trying to increase the bioavailability of curcumin different formulations were designed. Among them, nanoglobules based nanoemulsion formulation showed a hight release than suspension curcumin. Another study showed an encapsulating the curcumin into the hydrogel nanoparticles yielded a homogenous curcumin dispersion in aqueous solution compared to the free form of curcumin.The pharmacokinetics of curcumin and another formulation nanoemulsion curcumin (NEC) containing up to 20% curcumin (w/w) showed a 10 fold increase in the area under the blood concentration-time curve (AUC) 24 hours and more than 40-fold increase in the C(max) in NEC compared to curcumin in mice.
Another curcumin-loaded apotransferrin nanoparticles (nano-curcumin), prepared by sol-oil chemistry, releases significant quantities of drug gradually over a fairly long period, ~50% of curcumin still remaining at 6 hours of time. In contrast, intracellular soluble curcumin (sol-curcumin) reaches a maximum at 2 hours followed by its complete elimination by 4 hours.
The colloidal nanoparticles, named as 'theracurmin' showed AUC after the oral administration more than 40-fold higher than that of curcumin powder in rats. In healthy human volunteers, theracurmin (30 mg) when administered orally resulted 27-fold higher AUC than that of curcumin powder.
The nanoparticle of curcumin prepared by Cheng et al. produced significantly higher curcumin concentration in plasma and six times higher AUC and mean residence time in mice brain than regular curcumin. Thus, nanocurcumin enhances bioavailability of curcumin in animals as well as in humans.
Polylactic-co-glycolic acid (PLGA)
To improve the pharmacokinetics of curcumin with enhancing its bioavailability other effective formulation PLGA encapsulated curcumin was prepared.Intravenous administration of either curcumin or PLGA-curcumin (2.5 mg/kg), exhibited almost twice as high serum concentration of PLGA-curcumin than curcumin.
In support of previous study, it has been found that after oral administration of curcumin-PLGA-nanoparticles, the relative bioavailability was increased 5.6-fold and has a longer half-life compared with that of native curcumin. This improved oral bioavailability of curcumin found to be associated with improved water solubility, higher release rate in the intestinal juice, enhanced absorption by improved permeability, inhibition of P-glycoprotein-mediated efflux, and increased residence time in the intestinal cavity.
Liposomal encapsulation
Another formulation designed for improvement of bioavailability of curcumin is liposomal curcumin. Liposomes are considered as effective drug carriers because of their ability to solubilize hydrophobic compounds and to alter their pharmacokinetic properties.In rat oral administration of liposome-encapsulated curcumin (LEC) showed high bioavailability of curcumin. In addition, a faster rate and better absorption of curcumin were observed as compared to the other forms. Oral LEC gave higher C(max) and shorter T(max) values, as well as a higher value for the AUC, at all time points.
Cyclodextrin (CD)
CD, cyclic oligosaccharides, has been also used in order to improve curcumin's delivery and bioavailability via its encapsulation with CD. It has been found that CD encapsulated curcumin (CDC) had a greater cellular uptake and longer half-life in the cancer cells compared with free curcumin indicating CDC has superior attributes compared with free curcumin for cellular uptake. In addition, the improvement of CUR permeability acrossed animal skin tissue was observed in CD encapsulated curcumin and was about 1.8-fold when compared with the free curcumin. Thus, these studies suggest that CDC has improved in vitro and in vivo bioavailability and chemotherapeutic efficacy compared to curcumin alone.Piperine
Besides these natural compounds have been also used to increase the bioavailability of curcumin. One of them is piperine, a major component of black pepper, known as inhibitor of hepatic and intestinal glucuronidation and is also shown to increase the bioavailability of curcumin. This effect of piperine on the pharmacokinetics of curcumin has been shown to be much greater in humans than in rats. In humans, curcumin bioavailability was increased by 2,000% at 45 minutes after co-administering curcumin orally with piperine, whereas in rats, it has been found that concomitant administration of piperine 20 mg/kg with curcumin 2 g/kg increased the serum concentration of curcumin by 154% for a short period of 1-2 hours post drug. The study shows that in the dosages used, piperine enhances the serum concentration, extent of absorption and bioavailability of curcumin in both rats and humans with no adverse effects.In view of these findings, curcumin-piperine (Cu-Pi) nanoparticles has been prepared by various methods. The bioavailability, cellular uptake and biological effects of this nanoparticles are being tested.
Micellar curcumin
Although piperine boots absorption of curcumin it does not significantly delay its the elimination from the organism. Inhibiting curcumin metabolism by adjuvants (e.g. piperine) has small effect on circulating curcumin. Inhibiting phase II enzymes carries the additional risk of drug interactions.Despite its widespread coverage on internet discussion boards and in marketing campaigns of some companies, inhibiting phase II enzymes is a poor strategy to enhance curcumin bioavailability.
The use of micelles as carriers dramatically increases the absorption of curcumin for the body. In consequence, curcumin remains in the body at higher concentrations for loger after a single dose. Repeated intake results in significant curcumin concentrations in fasting plasma that exceed the maximum concentration reported in many pharmacokinetic trials. Curcumin can be quantified in brain tumors when administered as micellar formulation. This solution carries no adverse effects.
Sources
- Kunnumakkara AB, Harsha C, Banik K, Vikkurthi R, Sailo BL, Bordoloi D, Gupta SC, Aggarwal BB. Is curcumin bioavailability a problem in humans: lessons from clinical trials. Expert Opin Drug Metab Toxicol. 2019 Sep;15(9):705-733. doi: 10.1080/17425255.2019.1650914. Epub 2019 Aug 29. PMID: 31361978.
- Subramani PA, Narala VR. Challenges of curcumin bioavailability: novel aerosol remedies. Nat Prod Commun. 2013 Jan;8(1):121-4. PMID: 23472475.
- Jennifer Le , PharmD, MAS, BCPS-ID, FIDSA, FCCP, FCSHP, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California San Diego. Drug Bioavailability.
- Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, Zhai G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009 Apr 17;371(1-2):148-55. doi: 10.1016/j.ijpharm.2008.12.009. Epub 2008 Dec 13. PMID: 19124065.
- D.J. McClements et al. Boosting the bioavailability of hydrophobic nutrients, vitamins, and nutraceuticals in natural products using excipient emuls ions. Food Research International (2016).
- D.J. McClements et al. Current status in our understanding of physicochemical basis of bioaccessibility. Current Opinion in Food Science (2020).
- D.J. McClements. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnology Advances (2020).
- SamanSabeta, Ali Rashidinejadb, Laurence D. Meltonab, Duncan J.McGillivray. Recent advances to improve curcumin oral bioavailability. April 2021, Pages 253-266.
- Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92.
- Kumar A, Ahuja A, Ali J, Baboota S. Curcumin loaded nano globules for solubility enhancement: preparation, characterization and ex vivo release study. J Nanosci Nanotechnol. 2012;12:8293–8302.
- Guzman-Villanueva D, El-Sherbiny IM, Herrera-Ruiz D, Smyth HD. Design and in vitro evaluation of a new nano-microparticulate system for enhanced aqueous-phase solubility of curcumin. Biomed Res Int. 2013;2013:724763.
- Gandapu U, Chaitanya RK, Kishore G, Reddy RC, Kondapi AK. Curcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitro. PLoS One. 2011;6:e23388.
- Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660–665.
- Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer's disease Tg2576 mice. AAPS J. 2013;15:324–336.
- Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330–338.
- Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59:9280–9289.
- Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351:19–29.
- Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57:9141–9146.
- Yadav VR, Prasad S, Kannappan R, Ravindran J, Chaturvedi MM, Vaahtera L, et al. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol. 2010;80:1021–1032.
- Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin-beta-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech. 2013;14:1303–1312.
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356.
- Moorthi C, Krishnan K, Manavalan R, Kathiresan K. Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles. Asian Pac J Trop Biomed. 2012;2:841–848.
- Kocher A, Schiborr C. Superior oral bioavailability of micellar curcumin (2016).
